Crystal Field Theory
Crystal Field Theory: Overview
This Topic covers sub-topics such as Spectrochemical Series, Jahn Teller Effect, Strong Field Ligands, Crystal Field Splitting in Octahedral Complexes, Colour in Coordination Compounds and, Crystal Field Splitting in Tetrahedral Complexes
Important Questions on Crystal Field Theory
: [Ti(H2O)6]4+ is coloured while [Sc(H2O)6]3+ is colourless.
: d - d transition is not possible in [Sc(H2O)6]3+
The correct order for the wavelengths of absorption in the visible region for the following is:
The value of the spin only magnetic moment for one of the following configurations is BM. The correct one is
Given below are two statements, one is labelled as assertion and the other is labelled as Reason .
assertion : absorbs at lower wavelength of light with respect to
Reason : It is because the wavelenght of light absorbed depends on the oxidation state of the metal ion.
In the light of the above statements,choose the correct answer from the options given below:
Match List I with List II
| List I Complex | List II | ||
| A. | I. | ||
| B. | II. | ||
| C. | III. | ||
| D. | IV. |
Choose the correct answer from the options given below:
Which of the following complexes will exhibit maximum attraction to an applied magnetic field?
The homoleptic and octahedral complex of and has ______ unpaired electron(s) in the set of orbitals.
The complex with highest magnitude of crystal field splitting energy is
In potassium ferrocyanide, there are ______ pairs of electrons in the set of orbitals.
Match List-I with List-II.
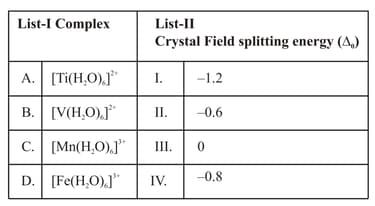
Choose the correct answer from the options given below:
The magnitude of crystal field stabilisation energy (CFSE) in tetrahedral complexes is considerably less than in the octahedral field because
CFSE of octahedral complex of (low spin) will be: [Excluding pairing energy term ]
Crystal Field Splitting Energy (CFSE) for is . The Crystal Field Splitting Energy (CFSE) for will be
The correct order of decreasing field strength of the below given ligands is
The crystal filed theory is successful in explaining which of the following?
I. Ligands as point charges;
II. Formation and structures of complexes.
III. colour;
IV. Magnetic properties:
V . covalent character of metal-ligand bonding.
Which of the following is correct related to the colours of
As is a strong ligand, then why it is not pairing -electrons in .
Hexacyano complexes of metals in their +2 oxidation state are usually yellow while the corresponding hexaaqua compounds are often blue or green, this is so because
Which of the following complex will absorb maximum wavelength of light?
Which of the following configuration of ions has zero CFSE in both strong and weak ligand fields ?
